Unidad de Investigación Clínica
Presentación
La Unidad de Investigación Clínica (UIC) es una Unidad Central de Apoyo a la Investigación Biomédica del IMIBIC. Constituye un área especializada cuyos objetivos principales son apoyar a los investigadores en el impulso y desarrollo de investigación clínica de calidad y garantizar los derechos, seguridad e integridad de los participantes en los estudios clínicos, la fiabilidad de los resultados obtenidos y la transparencia de los procedimientos.
Para lograr estos objetivos, la UIC cuenta con un equipo de profesionales multidisciplinar acreditado que proporciona una gestión integral de los estudios clínicos (asesoría científica, técnica, regulatoria, y metodológica) y una plataforma de soporte para la ejecución de ensayos clínicos en todas sus fases y otros proyectos de investigación.
Estructura Organizativa
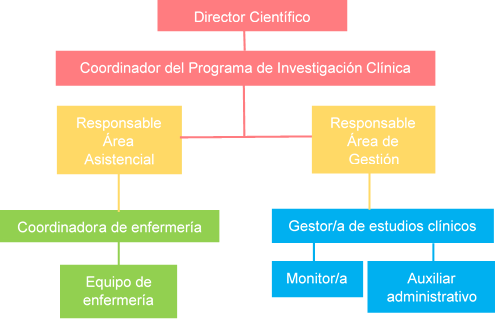
La UIC está formada por dos áreas:
- Área de Gestión: tiene como misión proporcionar apoyo y asesoramiento a los investigadores de nuestro centro en cuestiones éticas, metodológicas, regulatorias y de gestión para la realización y desarrollo de estudios clínicos propios aportando especialización, experiencia y conocimiento, asegurando que se realizan de acuerdo a las normas éticas y metodológicas establecidas y cumpliendo fielmente la normativa aplicable. Pertenece a la Plataforma de Apoyo a la Investigación Clínica Independiente SCReN (Spanish Clinical Research Network) del Instituto de Salud Carlos III.
- Área Asistencial: es una infraestructura dedicada en exclusiva a la investigación clínica que proporciona a los investigadores los recursos necesarios, tanto materiales como humanos, para la correcta ejecución de los ensayos clínicos y otros proyectos de investigación: consultas médicas, Hospitales de Día, laboratorios, equipamiento, enfermería especializada.
Estructura de la unidad
Quiénes somos

José Carlos Garrido Gracia
Responsable del Área de Gestión de la UIC
- josecarlos.garrido@imibic.org
- +34 677 906 567 (Corporativo 736567)
- +34 957 213 831 (Corporativo 3831)

Jaime Monserrat Villatoro
Responsable Área Asistencial de la UIC
- jaime.monserrat@imibic.org
- +34 683477861 (Corporativo 737943)