Investigación Clínica
Estudios Clínicos en el Hospital Universitario Reina Sofia
Los ensayos clínicos permiten realizar una evaluación experimental de un producto, sustancia, medicamento, técnica diagnóstica o terapéutica que, en su aplicación a seres humanos, pretende valorar su eficacia y seguridad.
Los pacientes tratados en el Hospital Universitario Reina Sofía, así como en los distritos sanitarios de la provincia de Córdoba son candidatos potenciales a participar en los ensayos clínicos desarrollados en sus instalaciones con el objetivo principal de que mejoren sus tratamientos.
Distribución de los ensayos clínicos activos por Servicio y Fases. Datos del 31 de diciembre 2024.
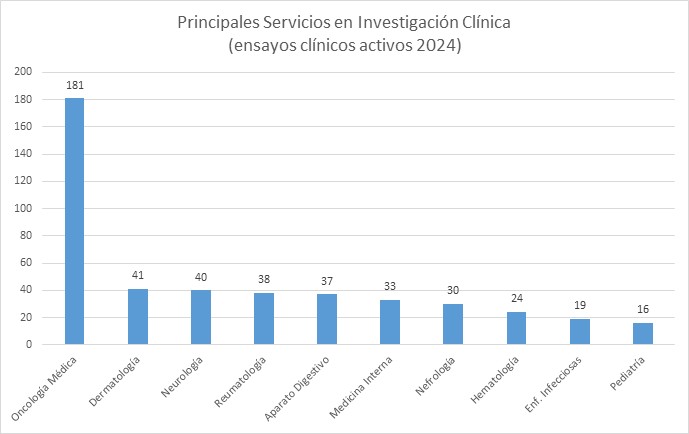
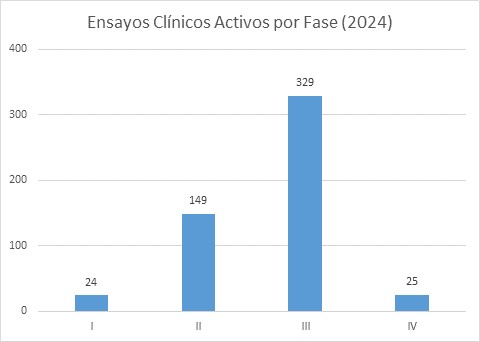
El IMIBIC dispone de un Clinical Trial Management System, que permite la monitorización a tiempo real de los datos e indicadores derivados de ensayos clínicos, en distintos aspectos, desde tiempos de firmas, inclusión de pacientes o reclutamiento. Además, también permite la comparación de los datos con los resultados publicados por farmaindustria a nivel nacional.
| INDICADORES | VALOR IMIBIC | VALOR NACIONAL |
|---|---|---|
| Tiempo desde el envío al CEIm de la documentación hasta la Inclusión del Primer Paciente *Días | 145 | 225 |
| Tiempo desde el envío al CEIm de la documentación hasta la última firma del Contrato *Días | 79,5 | 136 |
| Tiempo desde la recepción del Contrato Firmado a la Inclusión del Primer Paciente*Días | 44,5 | 71 |
| Tiempo de aprobación desde el envío de la documentación al CEIm hasta la aprobación del CEIm local *Días | 29 | 55 |
| Tasa de reclutamiento *% | 99% | 101% |
| Velocidad de reclutamiento *paciente/mes | 1,32 | 1,26 |