Investigación Clínica
Asistencial
El Área Asistencial es una infraestructura dedicada en exclusiva a la investigación clínica que proporciona a los investigadores los recursos necesarios, tanto materiales como humanos, para la correcta ejecución de los ensayos clínicos y otros proyectos de investigación: consultas médicas, Hospitales de Día, laboratorios, equipamiento, enfermería especializada.
Desde el inicio de su actividad en el año 2014, en esta área han sido atendidos más de 3.000 pacientes participantes en 200 estudios clínicos de diversas fases (desde fases tempranas a fases III). Diversos servicios médicos desarrollan sus estudios clínicos en esta área: Oncología Médica, Medicina Interna, Dermatología, Nefrología, Enfermedades Infecciosas, Reumatología, Urología, Aparato Digestivo, Endocrinología, Alergología y Pediatría.
Situadas en el complejo hospitalario Reina Sofía ocupan una superficie total aproximada de 2000 metros cuadrados y están distribuidas en dos instalaciones totalmente adaptadas y renovadas:
- Edificio de Investigación Clínica IMIBIC
- Hospital Provincial, primera planta ala izquierda
Cada una de ellas dispone de todo el material y sistemas necesarios para el desarrollo de los diferentes estudios clínicos:
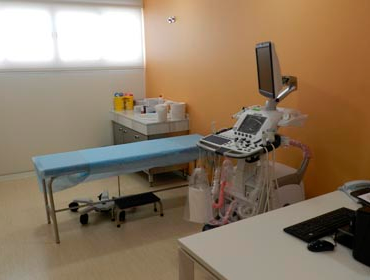
- Consultas médicas, un total de 9, equipadas con todo lo necesario para el seguimiento y exploración de los pacientes
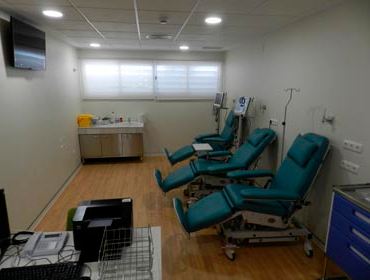
- Dos Hospitales de Día para adultos, con un total de 14 sillones articulados y 3 camas, dotados de control de enfermería, equipos instrumentales de monitorización, sistemas de reanimación avanzada, medicación de soporte y sistemas audiovisuales
- Recepción y sala de espera para participantes y familiares
- Sala de estar para pacientes
- Laboratorios para el procesado de muestras biológicas, dotado de centrifugas con control de temperatura
- Equipos de frío (refrigeradores, congeladores y ultra congeladores) con sistema de alarma vigilada y control de temperatura centralizado en el hospital
- Almacenes para material y fungibles
- Archivo de documentación con medidas de protección contra incendios
- Despachos para la gestión de datos y monitorización de estudios con ordenadores, faxes y teléfonos
- Equipamiento básico con electrocardiógrafos, monitores de presión arterial, básculas con tallímetros y pulsioxímetros
La unidad ha adquirido un Sistema de ultrasonido Logiq E9 que combina en tiempo real el ultrasonido con imágenes de ultrasonido u otras modalidades, como la tomografía o la resonancia, previamente realizadas.
La sala de usos múltiples es un espacio amplio y polivalente con capacidad para realizar presentaciones, reuniones de investigadores, actividades grupales, etc. Una parte se encuentra habilitada como gimnasio, con el equipamiento necesario para recoger parámetros de actividad física.
Destaca el área dedicada a la investigación clínica Pediátrica. Esta zona, ubicada en la planta baja del Edificio de Investigación Clínica IMIBIC, dispone de un acceso y recepción independiente, aseos, una consulta médica y un Hospital de Día totalmente equipado y adaptado a esta población.
Los investigadores interesados en el uso de estos recursos deben seguir el circuito establecido y aportar la documentación necesaria descrita en el Documento sobre uso del Área asistencial y el Formulario de Solicitud de Servicios.
El equipo de enfermería de la Unidad de Investigación Clínica está constituido por un conjunto de profesionales especializados cuyos objetivos principales son proporcionar una atención enfermera segura y de calidad a los participantes en los estudios clínicos y garantizar la correcta implementación de los diversos protocolos. Para ello, colaboran directamente con los equipos multidisciplinarios de investigación (investigadores, coordinadores de estudios, gestores de datos) de los servicios médicos que desarrollan sus estudios en la unidad.
El personal de enfermería está acreditado en Normas de Buena Práctica Clínica y Soporte Vital Avanzado. Posee experiencia en una gran variedad de protocolos de investigación clínica, en especial ensayos clínicos oncológicos y administración de tratamientos citostáticos.
Actividades:
- Administración de la medicación en investigación de acuerdo a los protocolos de los estudios
- Extracciones sanguíneas y manejo de muestras
- Electrocardiogramas (ECG)
- Registro de parámetros antropométricos (peso, talla) y toma de constantes (tensión arterial, temperatura, frecuencia cardiaca)
- Observación de los participantes durante su ingreso en la unidad y tratamiento de los síntomas
- Valoración inicial y cuidados de enfermería
- Consulta y seguimiento telefónico de los pacientes
- Recopilación de datos de calidad
Equipo
Nurses
- Coordinadora: Marta Checa Expósito
- Juan María Marín Fernández
- Mª Dolores Díez Ruano
- Miguel Puentes Aguado
- Carmen María Jiménez Ríos
- Maria Estela Mayen Jiménez
Assistant nurses
- Inmaculada Gálvez Membrives
- Elena Parrado González
- Rubén Sánchez Nieves
El área de ensayos clínicos del Servicio de Farmacia sirve de apoyo a los investigadores y garantiza un adecuado control, gestión, almacenamiento y conservación de las muestras de medicamentos en investigación y el acceso a la información de los medicamentos objeto de estudio.
Actividades
- Recepción, custodia, conservación y control del inventario de las muestras
- Preparación, dispensación, manejo y control de las muestras
- Devolución de la medicación sobrante a la finalización del estudio
- Mantenimiento de los registros de movimiento de la medicación
- Elaboración de normas y procedimientos escritos
Equipo de Farmacia

María José Gómez Luna
- mariaj.gomez@imibic.org
- 957010228
- 957011450

Rafael Serrano Berzosa
- rafael.serrano@imibic.org
- 957010228
- 957011450
Persona de contacto

Jaime Monserrat Villatoro
Responsable Área Asistencial de la UIC
- jaime.monserrat@imibic.org
- +34 683477861 (Corporativo 737943)
Instalaciones