Unidades Centrales de Apoyo a la Investigación Biomédica
Citometría de flujo y Microscopía óptica avanzada
La Unidad de Citometría de flujo y Microscopía Óptica Avanzada del IMIBIC comprende dos áreas tecnológicas de gran importancia en el campo de la investigación biomédica: la Citometría de flujo y la microscopía Confocal-fluorescencia.
Su misión es poner a disposición de los investigadores del IMIBIC y de instituciones públicas o privadas, un equipamiento especializado que cubra un amplio espectro de aplicaciones en estas áreas, además de proporcionar experiencia técnica y formación. La Unidad también proporciona asesoramiento y soporte científico-técnico sobre diseño experimental, preparación de muestras, análisis de datos de citometría, procesamiento y análisis de imagen, e interpretación de datos.
Personal

Esther Peralbo Santaella, PhD
Responsable de Unidad. Especialista Técnico de Citometría de flujo
- citometria@imibic.org
- (+34) 957 213 752 (Corporativo 3752)

Gema García Jurado, PhD
Responsable de Unidad. Especialista Técnico de Microscopía
- gema.garcia@imibic.org
- microscopia@imibic.org
- (+34) 957 213 753 (Corporativo 3753)
- 609 15 77 90
Equipamiento e infraestructuras
ÁREA DE CITOMETRÍA DE FLUJO
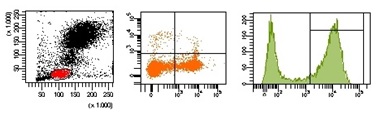
Fundamentos de la Citometría de flujo
La Citometría de flujo es una potente herramienta que permite medir rápida y simultáneamente, múltiples
características físicas y químicas de partículas únicas en suspensión, normalmente células, cuando se mueven
por una corriente de fluido a través de un haz de luz láser. Las propiedades medidas incluyen tamaño
relativo, granularidad o complejidad interna relativas, e intensidad de fluorescencia relativa de las
partículas. Estas características son determinadas mediante un sistema de acoplamiento óptico-electrónico
que analiza cómo cada célula o partícula dispersa la luz láser incidente y emite fluorescencia.
El Cell Sorting es un tipo especializado de citometría de flujo, que permite separar o aislar célula a
célula, subpoblaciones celulares presentes en una suspensión heterogénea, basándose en las características
específicas de dispersión de la luz y fluorescencia de cada una de ellas.
EQUIPAMIENTO
Analizadores:
- LSR Fortessa SORP (Becton Dickinson) (Última actualización: 2017): Equipado con 4 láseres (405 nm, 488 nm, 561 nm y 640 nm). Permite el análisis simultáneo de 20 parámetros, 18 fluorescencias más los parámetros de dispersión FSC y SSC. (optical configuration)
- Cytomics FC500 MCL (Beckman Coulter): Equipado con 2 láseres (488 nm y 635 nm). Permite el análisis simultáneo de 7 parámetros, 5 fluorescencias más los parámetros FSC y SSC. Equipado con cargador carrusel automático de 32 tubos. (optical configuration)
- FACSCalibur (Becton Dickinson): Equipado con 1 láser (488 nm). Permite el análisis simultáneo de 5 parámetros, 3 fluorescencias más los parámetros FSC y SSC.(optical configuration)
Separador (cell sorter):
- FACSAria III (Becton Dickinson). (optical
configuration) (Última actualización: 2014)
Características:- Equipado con 3 láseres (407 nm, 488 nm y 633 nm). Permite el análisis simultáneo de 13 parámetros, 11 fluorescencias más los parámetros FSC y SSC.
- Nozzles disponibles: 70µm, 85µm y 100µm.
- Capacidades de separación simultánea de hasta 4 subpoblaciones y célula única. Las células separadas pueden ser recogidas en tubos (microtubos, 12 x 75 mm and 15 mL), placas (6, 24, 48, 96 y 384 pocillos), o sobre portas (equipado con ACDU).
- Control de temperatura en el punto de inyección de la muestra (software ajustable), y en el punto de recogida de las células separadas (unidad de recirculación de agua).
- Equipado con sistema de evacuación de aerosoles (AMO: Aerosol Management Option).
APLICACIONES
Existe un amplio rango de aplicaciones en Citometría de flujo, entre éstas se encuentran:
- Análisis multicolor de antígenos de superficie e intracelulares (inmunofenotipaje)
- Viabilidad celular
- Ensayos de apoptosis (integridad de la membrana plasmática, función mitocondrial, actividad caspasa, fragmentación del ADN, etc.)
- Análisis de contenido de ADN y ciclo celular
- Ensayos funcionales como: proliferación celular (CFSE), flujos de Calcio, cambios de pH intracelular, producción de ROS, etc
- Análisis de expresión génica mediante genes reporteros
- Ensayo PrimeFlow RNA
- Análisis de microvesículas
- Análisis de microorganismos
- Separación y aislamiento de subpoblaciones celulares, y clonaje celular (Cell Sorting)
ÁREA DE MICROSCOPÍA CONFOCAL-FLUORESCENCIA
Fundamentos de la Microscopía confocal
La Microscopía confocal ofrece varias ventajas sobre la microscopía de fluorescencia widefield convencional. Éstas incluyen: la capacidad de controlar la profundidad de campo, la eliminación o reducción de las señales luminosas (background) procedentes fuera del plano focal, lo que incrementa la resolución y contraste de las imágenes, y la capacidad para obtener secciones ópticas seriadas de la muestra, lo que permite realizar análisis tridimensionales de la misma y observar preparaciones más gruesas. El principio de la Microscopía confocal se basa en el uso de técnicas de filtrado espacial para eliminar la luz fuera de foco de la muestra. Así, un haz de luz láser es enfocado en un punto de escaneo a una profundidad específica dentro de la muestra. Ello produce una emisión de fluorescencia, y mediante un diafragma (pinhole) situado en un plano conjugado al plano focal del objetivo en la muestra (confocal), se permite que sólo las señales fluorescentes procedentes del punto iluminado sean recogidas por el detector, mientras que, las señales luminosas procedentes de planos superiores e inferiores al plano focal de interés son bloqueadas. De esta manera, mediante un patrón de escaneo de la muestra por el punto de luz láser, se obtienen imágenes de un único plano óptico.
EQUIPAMIENTO:
- Microscopio confocal espectral LSM 710 (Carl Zeiss) (Última actualización: 2017)
Características:- Estativo: microscopio Axio Observer.Z1 invertido motorizado, con sistema de iluminación para campo claro, DIC y epifluorescencia.
- Objetivos:
- Plan-Apochromat 25x/0.8 Imm DIC
- C-Apochromat 40x/1.1 W DIC
- Plan-Apochromat 40x/1.3 Oil DIC
- Plan-Neofluar 63x/1.3 Imm DIC
- Plan-Apochromat 63x/1.46 Oil
- Láseres de excitación: láser Ar (458, 488, 514 nm), láser HeNe (543 nm), láser HeNe (594 nm), láser HeNe (633 nm) y láser diodo violeta (405 nm).
- Detección: 3 canales de detección espectral de fluorescencia con PMTs, y 1 canal para luz transmitida con PMT y capacidad DIC.
- Sistema de incubación celular integrado XL.
- Microscopio confocal LSM 5 Exciter (Carl Zeiss).
Características:- Estativo: microscopio Axio Observer.Z1 invertido motorizado, con sistema de iluminación para campo claro y epifluorescencia.
- Objetivos:
- Plan-Neofluar 10x/0.3
- Plan-Neofluar 20x/0.5
- Plan-Neofluar 40x/1.3 Oil
- Plan-Apochromat 63x/1.4 Oil DIC
- Láseres de excitación: láser Ar (458, 488, 514 nm), láser HeNe (543 nm), láser HeNe (633 nm) y láser diodo violeta (405 nm).
- Detección: 2 canales de detección de fluorescencia con PMTs.
- Mini-incubador celular adaptable a platina.
- Microscopio de fluorescencia Axio Vert.A1 (Carl Zeiss).
Microscopio invertido con sistema de iluminación para campo claro y epifluorescencia. Objetivos: A-Plan 20x/0.35, Plan-Neofluar 40x/0.9 and Plan-Neofluar 63x/1.25 oil. Cubos de filtros para DAPI, GFP/FITC y Cy3/Texas Red. Equipado con una cámara CCD monocroma AxioCam ICm1-1.4 Mpx, y software ZEN 2 (Zeiss) para la adquisición de imágenes.
APLICACIONES
Las principales aplicaciones de la microscopía confocal incluyen:
- Análisis y tracking de estructuras subcelulares
- Estudios de colocalización
- Obtención de secciones seriadas en Z e imagen 3D
- Adquisición multidimensional de imágenes (x, y, z, t y lambda stack)
- Adquisición de imágenes en mosaico
- Análisis FRET para el estudio de interacciones moleculares
- Análisis de Dinámicas moleculares en célula viva mediante FRAP y FLIP
- Microscopía "in vivo"
Servicios
- Uso de los citómetros de flujo analizadores y equipos de microscopía en régimen de autoservicio (sólo usuarios internos debidamente entrenados por el personal técnico).
- Uso asistido por operador técnico del equipamiento.
- Cell Sorting: servicio sólo proporcionado con operador técnico.
- Procesamiento y análisis de imagen. Análisis de datos de citometría de flujo.
- Soporte y asesoramiento en relación a: diseño experimental, protocolos de preparación de muestras, interpretación y presentación de datos.
- Desarrollo/optimización de ensayos/aplicaciones de citometría y microscopía así como, de herramientas de análisis de imagen, en respuesta a las necesidades de los usuarios.